Abstract
Background. Despite advances in therapy, treatment of R/R NHL remains a challenge. PEVO (TAK924) is a first-in-class, selective, small molecule, NAE inhibitor with preclinical activity in NHL, including mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL) and diffuse large B cell lymphoma (DLBCL). We and others have demonstrated synergy between PEVO and the Bruton tyrosine kinase inhibitor ibrutinib, in in vitro and in vivo models of CLL and MCL. We report the results of a phase 1 study of PEVO in combination with ibrutinib in patients with R/R NHL.
Methods. This was a multicenter, open-label, Phase I, investigator-sponsored study (NCT03479268). Patients with NHL or CLL ≥18 years of age whose disease had relapsed or was refractory to ≥1 prior lines of therapy were enrolled. The Phase I portion of the study followed mTPI design with five planned dose levels of PEVO (15, 20, 25, 37.5 and 50 mg/m 2). PEVO was given IV on days 1, 3 and 5 of a 21-day cycle for a total of 8 cycles. Ibrutinib was administered once a day starting on Cycle 1 day 2 at a fixed dose of 420 mg (560 mg in MCL) orally for a total of 18 cycles. Primary study objectives were safety and tolerability; secondary objectives included preliminary measures of efficacy. Response was assessed by Lugano (NHL) and IWCLL criteria. Exploratory objectives included pharmacokinetic (PK) and pharmacodynamic endpoints. Dose limiting toxicities (DLT) were defined as grade 4 hematologic or grade 3 non-hematologic toxicities.
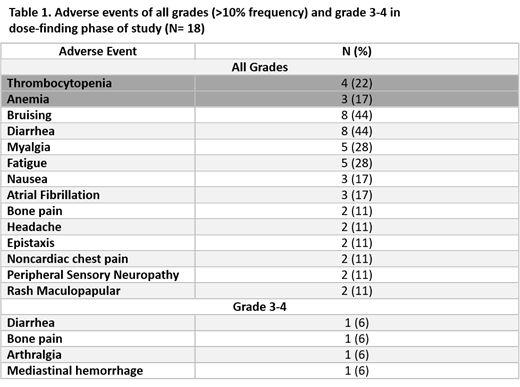
Results. Of the eighteen patients enrolled, 8 had MCL, 4 had DLBCL, 4 had CLL and one patient each had marginal zone and follicular lymphoma; 72% (13/18) were men. Median age was 71 (51-90) years, 94% had an ECOG performance status ≤1. Patients had received a median of 1 prior line of therapy (range, 1-3), 3 patients had undergone prior autologous stem cell transplant. Three patients each were treated on dose level cohorts 1-4. One DLT, mediastinal hemorrhage, was observed at dose level 5. Therefore, the recommended phase 2 dose (RP2D) of PEVO was 50 mg/m 2. The most common treatment-related adverse events (AEs, any grade) were bruising and diarrhea (44% each; Table 1). There was one case each (5.6%) of the following grade 3-4 AEs: arthralgia, bone pain, diarrhea and mediastinal hemorrhage. Three patients (16.6%) developed grade 1-2 atrial fibrillation on study but all continued therapy. Treatment discontinuation due to AE occurred in one patient (5.6%, mediastinal hemorrhage). The overall response rate (ORR) was 66% for all patients. Among eight patients with MCL, ORR was 100% (50% complete response). The median PEVO elimination T 1/2 was 6.7 h (range, 4.9-12.1 h) on day 1 and 6.4 h (3.2-11.0 h) on day 3 (Wilcoxon p=0.12). PEVO exhibited linear PK with dose-dependent increases in C max and AUC (0-inf) (Figure 1), which were unaffected by concurrent ibrutinib (Wilcoxon p=0.09 and 0.94, respectively).
Discussion. The combination of pevonedistat and ibrutinib is safe and well-tolerated in patients with R/R NHL. It is associated with high response rates in patients with R/R MCL. The study is currently enrolling patients in the expansion cohort to further characterize the efficacy of this promising regimen.
Torka: TG Therapeutics: Membership on an entity's Board of Directors or advisory committees. Herrera: Bristol Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Karyopharm: Consultancy; Takeda: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Tubulis: Consultancy; Seagen: Consultancy, Research Funding; Kite, a Gilead Company: Research Funding; Merck: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Gilead Sciences: Research Funding. Hernandez-Ilizaliturri: AbbVie: Other: Advisory Boards; Incyte: Other: Advisory Boards; Celgene: Other: Advisory Boards; BMS: Other: Advisory Boards; Pharmacyclics: Other: Advisory Boards; Amgen: Other: Advisory Boards; Kite: Other: Advisory Boards; Gilead: Other: Advisory Boards; Epyzime: Other: Advisory Boards. Danilov: SecuraBio: Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Bayer Oncology: Consultancy, Honoraria, Research Funding; Takeda Oncology: Research Funding; Rigel Pharm: Honoraria; TG Therapeutics: Consultancy, Research Funding; Gilead Sciences: Research Funding; Bristol-Meyers-Squibb: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria, Research Funding.
pevonedistat - clinical trial

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal